Bridgemed Solutions, Inc.
We are the leading Contract Manufacturing Organization (CMO) in the transcatheter heart valve area. We custom produce Transcatheter Aortic Valves (TAVR), Transcatheter Mitral Valves (TMVR) and Transcatheter Tricuspid Valves as per client requirements based on their IP.
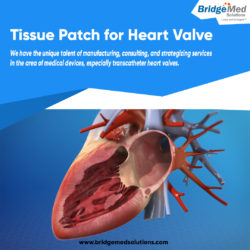
Tissue Patch for Heart Valve is used by doctors during heart surgery to fix holes or tears in the valve. This tissue patch is available at Bridgemed and is created under the supervision of experts using high-quality materials.
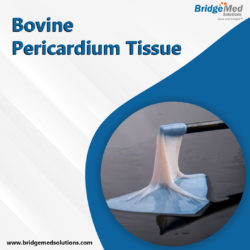
Bridgemed Solutions, Inc. is the world’s leading provider of patient-centered medical breakthroughs for structural heart disease, critical care, and surgical monitoring.

The product was assessed and the ISO-10993 and FDA G#95 memorandum requirements were reviewed by a BridgeMed solutions expert. The tests advised by the labs, according to the research, are off-the-shelf assays that are not personalised to the product.

Bridgemed continues to work with a dedication to give cardiac surgeons with solid solutions from a firm they can trust as a pioneer in heart valve therapy.
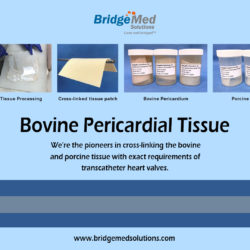
This project was examined by a BridgeMed solutions consultant, who compared end-to-end manufacturing for impacted products, identified manufacturing steps for which rationale could be written, identified manufacturing for which testing was required, developed strategies to demonstrate compliance, and assisted in the preparation of regulatory documents for submission
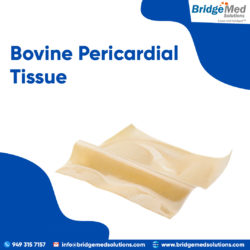
Pericardial tissue and Elgiloy stent material are both known for their optimal intrinsic properties for valve manufacture. It combines the latest engineering advancements with the benefits of bovine pericardial tissue. TISSUE PATCH FOR SALE
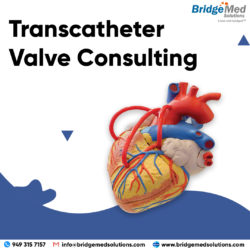
We provide Transcatheter Valve Consulting and tailor our solutions to the medical device, saving our clients both time and money. Call us today at 949-315-7157. Consulting Services

We provide patients with access to a team of specialists at Bridgemed Solutions for Transcatheter Valve Therapy that can help diagnose. Our specialists are experts in the most up-to-date treatment methods, including minimally invasive catheter-based procedures, and provide each patient with an individualized treatment plan. Transcatheter Heart Valve Therapies
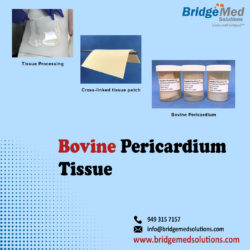
We were the first to cross-link bovine and porcine tissue to meet the exact specifications of transcatheter heart valves. We offer glut fixed GBR1 pericardium tissue from bovine, porcine, and equine sources for aortic, mitral, pulmonic, and tricuspid valves, as well as a variety of surgical applications. TISSUE PATCH FOR SALE boardboardboardboardboardboardboardboardboardboardboardboardboardboardboardboardboard
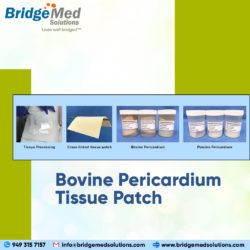
Bridgemed Solutions offers a variety of bovine pericardium tissue harvesting and selection options to meet manufacturers requirements. Please contact us to discuss your requirements for Bovine Pericardium Tissue Patch. TISSUE PATCH FOR SALE
Bridgemed is a preclinical center of excellence for medical device interventional research, offering cutting-edge equipment and interventional techniques. Our experts are highly trained and experienced, having trained with some of the most prominent doctors and researchers in the world. tavr biocompatibility studies

Its breathable material and its cut with raglan sleeves and flatlock stitching make this product ideal for all your sports operations. It is available in a wide range of sizes and colours, also in neon tones.

The efficacy of anti-calcification treatment of bioprosthetic heart valves remains unclear. We used urazole and glutamate to neutralize the free aldehyde and some solvents to reduce the phospholipid content in the bovine pericardial tissue.

Bridgemed is a leading biological products company supplying animal and human tissues. We offer top quality, fully customized biological products and services at a best price. Give us a call on this number 949-315-7157. TISSUE SERVICES

Bridgemed creates cost effective contract manufacturing solutions to increase their clients flexibility and contributes to their overall profitability. Contact us for any inquiry! Contract Manufacturing Services

If your medical device is in any other class apart from class I, you will have to provide the Notified body with proof that your product fulfils the essential requirements of the respective CE directives. The medical devices of Class III hold the highest risk.
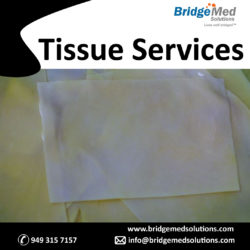
We provide Tissues Services for the heart valve industry at best prices. We procure bovine and porcine tissue for use in heart valve leaflets. boardboardboardboardboard


